Professor Richard Haverkamp and Dr Aaron Marshall are chemical engineers at Massey University, Palmerston North. They have created nanoparticles and catalysts that will help fuel1 the future. To understand their work, you’ll need to know a little about hydrogen2 fuel cells and catalysts.
Hydrogen fuel cells
Hydrogen could replace the burning of carbon-based fuels such as gas and oil, which create pollutants that include carbon dioxide3.
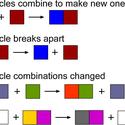
Hydrogen fuel cells combine the gases4 hydrogen and oxygen to create electricity5. Oxygen6 is got from air, but the hydrogen needs to be more pure. At the moment, the main sources of hydrogen are oil or natural gas, which are non-renewable resources. There is another source of hydrogen that is much more environmentally friendly – water.
When an electric current7 is passed through water, the hydrogen and oxygen from which it is made are split apart and can be collected. This process is called electrolysis8.
So, you use electricity to get hydrogen from water, then combine hydrogen and oxygen to make electricity and water. That sounds like a waste of energy, so why bother?
The electricity used to get hydrogen from water can be from renewable sources, such as solar or wind power. The hydrogen becomes a mobile source of energy. This makes it suitable for fuel cells, which can be used to power9 portable equipment when electricity is hard to get, such as in remote places. For example, fuel cells powered the electrical systems of NASA’s space shuttle10. Water is produced as a waste product of fuel cells, and this is easily recycled – in the case of the space shuttle, the crew used the waste water from fuel cells for drinking.
Speeding up the reaction – electrocatalysts
Electrocatalysts make the electrolysis of water much more efficient, so more energy ends up as hydrogen. One of the problems with the electrolysis of water is that, without chemical help, a lot of energy is wasted. This is where electrocatalysts come in. An electrocatalyst11 is a substance that speeds up a chemical reaction12 that uses or makes electricity.
Aaron Marshall and Richard Haverkamp have been very successful in developing electrocatalysts that greatly reduce the power needed to release hydrogen from water. They have engineered nanoparticles with a shape and composition that makes them very efficient electrocatalysts. In fact, with an efficiency of 76%, their electrocatalysts are world leaders.
The best catalysts are chemically active, last a long time and have a structure with a large surface area13 that allows lots of atoms to take part in the reaction. Making or improving a catalyst14 is a chemical balancing act of these aspects.
Nature of science
Many of the scientists working in nanotechnology15 today had never heard of the subject when they were at school – it is too new – so most scientists have come to nanotechnology from other topics, such as chemistry, physics, engineering, biology16, medicine and mathematics. Things have changed, and now you can study nanotechnology at University.
For example, nanoparticles of the metal17 ruthenium18 make a very active catalyst, but they are not very stable and do not have a large surface area. Aaron has been adding other metals to ruthenium, in the search to find an electrocatalyst that is active, has a stable structure and a high surface area. Aaron has achieved his best results using a mixture of iridium19, ruthenium and tantalum20, which are all transition metals21 on the periodic table22.
A new approach to nanoparticle structure
When a number of atoms group together to form a ball-shaped nanoparticle23, only the atoms on the outside are available to take part as catalysts in chemical reactions. Atoms on the inside of the particle24 are in effect wasted, and with material such as ruthenium and iridium, this can be a very expensive waste.
Aaron and Richard are experimenting with structures called core-shell particles. These have a core of cheap, non-reactive material (such as tin25 oxide26), surrounded by a thin shell of very reactive27 electrocatalyst. Most of the catalyst atoms are available for reaction, making these nanoparticles very efficient. And efficiency is everything!
Related content
In Gold nanoparticles from plants discover how gold28 and alloy29 nanoparticles being created in plants at Massey University could be used as catalysts in fuel cells for the military and other complex chemical reactions. Other researchers are making new nanoparticle shapes to increase the efficiency of catalysts and reduce poisonous30 emissions from car exhausts.
Chemical reactions and catalysts are two of the big science ideas that underpin nanoscience31 research. Use this activity to explore the nanoscience connection, students can use modelling clay to construct catalyst nanoparticle shapes and calculate surface area:volume ratios with the aim of trying to develop a more efficient shape.
Useful link
An article from the US Energy Information Administration about hydrogen and fuel cells.
- fuel: 1. A combustible substance that provides energy. 2. A body fuel such as fat, carbohydrates and protein that supplies energy for animals’ activities.
- hydrogen: First element on the periodic table – symbol H, with the atomic number of 1, meaning that it has a single proton in its nucleus.
- carbon dioxide: CO2 is a colourless, odourless, incombustible gas. It is a product of cellular respiration and combustion and is an essential component in photosynthesis.
- gases: The state of matter distinguished from the solid and liquid states. Gases have the ability to diffuse readily and to become distributed uniformly throughout any container.
- electricity: A general term that includes a variety of phenomena resulting from the presence and flow of electrical charge.
- oxygen: A non-metal – symbol O, atomic number 8. Oxygen is a gas found in the air. It is needed for aerobic cellular respiration in cells.
- current: The flow of electric charge through a conductor.
- electrolysis: A chemical change caused by passing electricity through a solution.
- power: 1. The rate at which work is done (defined as work divided by time taken). 2. Mechanical or physical energy, force or momentum.
- space shuttle: A reusable NASA spacecraft that carries astronauts, space station material and satellites into a low orbit around Earth.
- electrocatalyst: A substance that increases the rate of a chemical reaction that makes or uses electricity.
- chemical reaction: A process in which one or more substances are changed into different substances.
- surface area: The total area of an object or surface.
- catalyst: A substance that increases the rate of a chemical reaction but is not permanently changed by that reaction. In living organisms enzymes are catalysts.
- nanotechnology: Understanding and working with matter at the scale of atoms and molecules. 1 nanometre is a millionth of a millimetre.
- biology: The science of living things.
- metal: Any of a category of elements that usually have a shiny surface, are generally good conductors of heat and electricity and can be melted or fused, hammered into thin sheets or drawn into wires (for example, copper).
- ruthenium: A transition metal in Group 8 of the periodic table – symbol Ru, atomic number 44.
- iridium: A transition metal in Group 9 of the periodic table – symbol Ir, atomic number 77.
- tantalum: A transition metal in Group 5 of the periodic table – symbol Ta, atomic number 73.
- transition metals: A group of hard, tough metal elements, which conduct electricity. Includes well known metals such as gold, silver, iron, copper and platinum.
- periodic table: The organisation of all known elements into groups with similar properties.
- nanoparticle: A particle that has at least one dimension of 100 nm or less. Nanoparticles tend to have different properties to the same material at a larger size.
- particle: A tiny piece of matter. A particle may refer to an atom, part of an atom, a molecule or an ion.
- tin: A metal – symbol Sn, atomic number 50.
- oxide: A chemical compound made up of oxygen combined with at least one other element. Most of the Earth’s crust consists of oxides.
- reactive: The relative ability of an atom or molecule to undergo a chemical reaction with another atom, molecule or compound.
- gold: A transition metal in Group 11 of the periodic table – symbol Au, atomic number 79.
- alloy: A mixture of a metal with one or more other elements to modify its metallic properties, for example, brass is an alloy of copper and zinc.
- poisonous: Capable of harming or killing by or as if by poison. A poisonous organism only delivers its toxins when eaten, touched or inhaled.
- nanoscience: The study of atoms, molecules and objects whose size is on the nanometre scale (1–100 nanometres).