Calcium carbonate is the principal mineral component of limestone. Its chemical and physical properties lie behind the modern-day uses of limestone as well as the unique limestone landscapes of the countryside.
Calcium carbonate – mineral forms
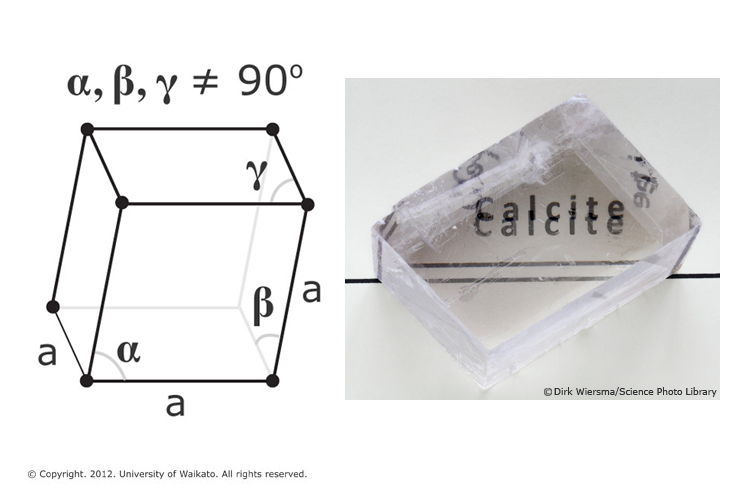
The principal mineral component of limestone is a crystalline form of calcium carbonate known as calcite. Although calcite crystals belong to the trigonal crystal system, shown below, a wide variety of crystal shapes are found.
Single calcite crystals display an optical property called birefringence (double refraction). This strong birefringence causes objects viewed through a clear piece of calcite to appear doubled.
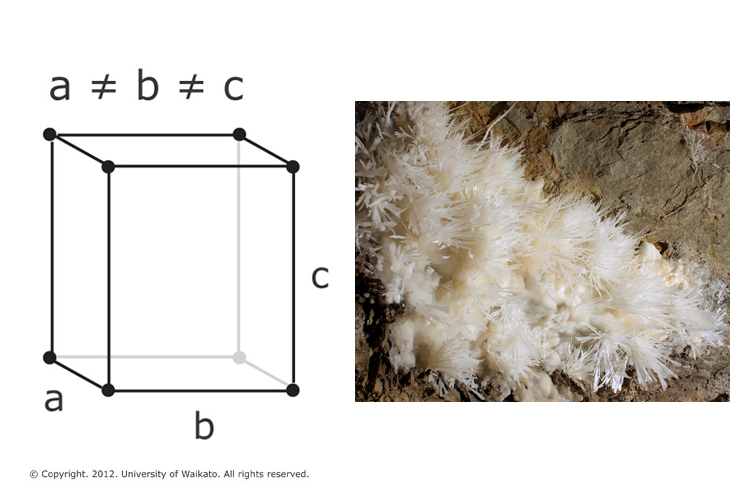
Another mineral form of calcium carbonate is called aragonite. Its crystal lattice differs from that of calcite, resulting in a different crystal shape – an orthorhombic system with needle-shaped crystals.
Solubility
Calcium carbonate has a very low solubility in pure water (15 mg/L at 25°C), but in rainwater saturated with carbon dioxide, its solubility increases due to the formation of more soluble calcium bicarbonate. Calcium carbonate is unusual in that its solubility increases as the temperature of the water decreases.
The increased solubility of calcium carbonate in rainwater saturated with carbon dioxide is the driving force behind the erosion of limestone rocks, leading to the formation over long periods of time of caverns, caves, stalagmites and stalactites. Rainwater is weakly acidic, and when it meets with limestone, some of the calcium carbonate reacts to form a solution of calcium bicarbonate.
Thermal decomposition
When heated above 840°C, calcium carbonate decomposes, releasing carbon dioxide gas and leaving behind calcium oxide – a white solid.
Calcium oxide is known as lime and is one of the top 10 chemicals produced annually by thermal decomposition of limestone.
The thermal decomposition of calcium carbonate to lime is one of the oldest chemical reactions known. For several thousand years, lime has been used in mortar (a paste of lime, sand and water) to cement stones to one another in buildings, walls and roads. The setting of mortar involves several chemical reactions.
First, the lime is ‘slaked’ by the water to produce calcium hydroxide (slaked lime
Over time, this reacts with carbon dioxide in the air to form crystals of calcium carbonate, which lock the sand grains together to form a hard rock-like material.
Reaction with acids
Like all metal carbonates, calcium carbonate reacts with acidic solutions to produce carbon dioxide gas. It is this reaction that is responsible for limestone fizzing when dilute hydrochloric acid is placed on its surface.
Limestone, which consists mostly of calcium carbonate, has been used in agriculture for centuries. It is spread on fields to neutralise acidic compounds in the soil and to supply calcium, which is an essential plant nutrient. Today, depending on the soil requirements, options available to the farmer are:
- lime – CaO
- slaked lime – Ca(OH)2
- crushed pure calcitic limestone – CaCO3
- dolomitic limestone – CaMg(CO3)2
In medicine, antacids containing small amounts of calcium carbonate are used in the treatment of ‘acid stomach’. The chemical reaction occurring involves the neutralisation of excess acid with calcium carbonate. Brands such as Quick-Eze and TUMS have calcium carbonate as the ‘active’ ingredient.
Nature of science
In trying to understand the world around us, scientists often look for patterns of behaviour that allow general rules or principles to be formulated. The watchful scientist, however, needs to have an open mind and know that there are always exceptions to the general rule, for example, where the solubility of calcium carbonate decreases with increasing temperature.