An interactive showing the main components of the terrestrial nitrogen cycle. Select one of the buttons to find out more.
Go here to view the full transcript and copyright information.

This interactive shows the main components of the terrestrial nitrogen cycle. Select one of the buttons to find out more.
Transcript
Nitrogen in the atmosphere
Lightning
Fossil fuel emissions
Volatilisation
Rain
Legumes
Produce
Fertilisers
Dung and urine
Run-off
Soil organic matter
Decomposition and mineralisation
Plant and microbial uptake
Nitrification
Nitrogen fixation
Leaching to groundwater
Denitrification
Soil nitrogen gas
Nitrogen in the atmosphere (N2)
About 80% of the atmosphere is dinitrogen gas, which is more or less unavailable to most plants. Nitrogen gas in the atmosphere is basically unusable by most of biology – plants and animals – but there are a few species of microbes in conjunction with plants that can convert the dinitrogen gas into usable forms of nitrogen like ammonium, and then that will turn into organic nitrogen or nitrate, and that can then enter the biological system.
The conversion of nitrogen gas into biologically available forms of nitrogen is critical for the functioning of the ecosystem.
ACKNOWLEDGEMENTS
Dr Kristine A Nichols
Professor Louis Schipper, University of Waikato
Lightning
Lightning storms are important for converting nitrogen gas in the atmosphere through to forms that are biologically available.
ACKNOWLEDGEMENTS
Image licenced through 123rf.com.
Fossil fuel emissions (NOx)
Fossil fuels are deposits of essentially animals or plants that have died in the past and become buried and then converted through to highly concentrated forms of energy like oil or coal.
If you think about plants and animals, both of them contain quite a lot of nitrogen, so when they get converted through to energy forms such as fossil fuels, that nitrogen is still contained in that fossil fuel. When you burn the fossil fuel, either say coal or oil, you are essentially releasing that nitrogen back up into the atmosphere – not necessarily as dinitrogen gas but maybe other forms of nitrogen that are more available to plants and animals.
So if that material goes up into the atmosphere, it can be rained out again and deposited on the land as a form of biologically available nitrogen.
Fossil fuels release nitrous oxide, which is one of the greenhouse gases that is released into the atmosphere, so fossil fuel burning does contribute to greenhouse gases, not only through the production of CO2 but also nitrous oxide.
ACKNOWLEDGEMENTS
Professor Louis Schipper, The University of Waikato Te Whare Wānanga o Waikato
Volatilisation (NH3)
Volatilisation is the conversion of dissolved ammonia – found in urine patches – into ammonia gas that can go into the atmosphere. This can happen if it’s hot, dry and windy. It is loss of nitrogen from the paddock to be avoided if possible. The rain may wash it back down but not necessarily to your farm. It could end up in a forest or out in the ocean.
ACKNOWLEDGEMENTS
The University of Waikato Te Whare Wānanga o Waikato
Rain (NOx, NH4+)
There are different nitrogen gases that come out of the soil. Ammonium, for example, will volatilise up into the atmosphere, fossil fuel burning will release nitrogen into the atmosphere and lightning will convert dinitrogen gas in the atmosphere to water-soluble forms of nitrogen. And when it rains, the rain will strip that nitrogen out of the atmosphere and deposit it onto the surface of the land.
ACKNOWLEDGEMENTS
Professor Louis Schipper, The University of Waikato Te Whare Wānanga o Waikato
Legumes
Legumes such as clover are incredibly important for nitrogen cycling, particularly in New Zealand. In the early days, when we didn’t have nitrogen fertilisers, they encouraged the growth of nitrogen fixers by adding phosphorous fertilisers. And the neat thing about clover is that it harbours a bacteria called rhizobia and houses them and encourages them to grow, and they will take dinitrogen gas out of the atmosphere and convert it through to ammonia and ammonium that the plant can then use. That nitrogen ultimately ends up getting into the soil and taken up by other plants. So clover growth has hugely increased the production of New Zealand pastures.
ACKNOWLEDGEMENTS
Professor Louis Schipper, The University of Waikato Te Whare Wānanga o Waikato
Damon Taylor
Professor Frank Dazzo
Produce
To make sure that you get good crop yields or grass yields, you need to make sure that you’ve got enough nitrogen for them to be able to build their proteins and the enzymes that they need. So without a good supply of nitrogen, it is hard to get large yields or grow the amount of food that you need. It is estimated that about 40% of the world’s population is fed by food that is grown using nitrogen that has been brought in either as fertiliser or through the deliberate cultivation of plant crops that are able to fix nitrogen out of the atmosphere.
ACKNOWLEDGEMENTS
Professor Louis Schipper, The University of Waikato Te Whare Wānanga o Waikato
David Higgitt http://creativecommons.org/licenses/by-sa/2.0/deed.en
Deeporaj http://creativecommons.org/licenses/by-sa/3.0/deed.en
Jan Ulrich http://creativecommons.org/licenses/by-sa/3.0/deed.en
Mark Silcock, McDonald’s Lime
Certain photos in this video are the copyrighted property of 123RF Limited, their contributors or licensed partners and are being used with permission under licence. These images and/or photos may not be copied or downloaded without permission from 123RF Limited.
Fertilisers
Fertilisers give farmers a tool to be able to boost production when they need to. If too much fertiliser is used or fertiliser is used at the wrong time of year, then it’s possible for farms to lose that nitrogen fertiliser either leaching down through the soil profile, or it can be converted through to nitrogen gas – as one of them being a greenhouse gas – which is what nobody really wants because it represents a loss of productivity. You are losing nitrogen off your farm, but you are exporting that nitrogen somewhere else where it can also act as a fertiliser, like in streams or in waterways, and that’s where you’ll get unwanted growth.
ACKNOWLEDGEMENTS
Mark Silcock, McDonald’s Lime
Juliet Milne, Otago Regional Council
Professor Louis Schipper, The University of Waikato Te Whare Wānanga o Waikato
Dung and urine
Urine in particular is very important for the cycling of nitrogen in pastoral systems. A cow grazes a large area and essentially concentrates all of that nitrogen that was in that grass into its body, and then when it has a urination event, it is depositing all of that nitrogen onto quite a small area.
The amount of nitrogen then in that little patch is way in excess of what the plants can handle, and you get nitrogen moving away from that site, because the plants basically can’t handle it. And you can see these urine patches when you drive past pastures. You’ll see these little tufts of green grass growing, and there are all these little patches out over the pasture. So if you ever go out for a drive and you go past a pasture and you see all of these little lumps – that’s old urine patches.
ACKNOWLEDGEMENTS
Professor Louis Schipper, The University of Waikato Te Whare Wānanga o Waikato
Run-off
Run-off is water that runs off over the surface of the land. If there’s too much rain and it’s greater than the rate that the soil can accept, it can pool on the surface, and if you have a slope, water will sheet over the surface of the land and go into waterways. Nitrate can be lost from soils through run-off.
ACKNOWLEDGEMENT
Public Domain
Soil organic matter
All of the nitrogen that is fixed out of the atmosphere goes into plants and then possibly into animals and then as those animals and plants die, they start to be decomposed, and that then enters into soil organic matter. So soil is a suite of different types of particles – sands, silts and clays – and some of the clays in particular are able to protect that organic matter from further decay.
So you get large build-ups of organic matter in soil, and that contains mainly nitrogen and carbon. There is more carbon in soils than in all the plants above the soil, and if you also add in the amount of carbon that’s in the atmosphere.
So there’s a tremendous amount of carbon in every hectare of land. For example, in New Zealand, it wouldn’t be unusual for a pasture to have something like 150 tonnes of carbon per hectare down to about a metre’s depth. Now along with that carbon is a lot of nitrogen, usually in a ratio about 10:1, so for every 10 tonnes of carbon, you’ll have about a tonne of nitrogen stored in the organic matter.
ACKNOWLEDGEMENTS
Professor Louis Schipper, The University of Waikato Te Whare Wānanga o Waikato
Barnowl
http://creativecommons.org/licenses/by-sa/2.0/deed.en
Certain photos in this video are the copyrighted property of 123RF Limited, their contributors or licensed partners and are being used with permission under licence. These images and/or photos may not be copied or downloaded without permission from 123RF Limited.
Decomposition and mineralisation
When nitrogen is fixed by plants and then is incorporated into their roots and leaves, that material will eventually die and will form part of the organic matter in soil, and that will start to degrade. It will be degraded by microorganisms in soil, and as that degradation occurs, nitrogen is released as nitrate or as ammonium, which then can be used by other forms of bacteria or plants. So mineralisation is the decomposition of litter in soil and the release of nitrogen into forms that plants can take up.
ACKNOWLEDGEMENTS
Professor Louis Schipper, The University of Waikato Te Whare Wānanga o Waikato
Certain photos in this video are the copyrighted property of 123RF Limited, their contributors or licensed partners and are being used with permission under licence. These images and/or photos may not be copied or downloaded without permission from 123RF Limited.
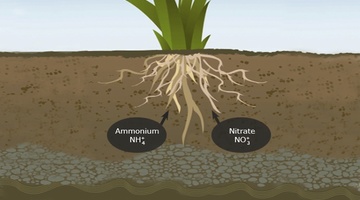
Plant and microbial uptake (NH4+, NH3-)
Plants and microorganisms can take up different types of nitrogen. The main ones are ammonium or nitrate. This is vital because nitrogen is critical for all life. It forms the backbone of most proteins or enzymes, and it’s also important part of DNA.
ACKNOWLEDGEMENT
The University of Waikato Te Whare Wānanga o Waikato
Nitrification
Nitrification is one of the steps in the nitrogen cycle where ammonium is converted to nitrate. First, ammonium is converted to nitrite by specialised bacteria. Other bacterial species are responsible for the conversion of nitrite to nitrate. Nitrate is easily taken up by plants, but it is also susceptible to loss because it doesn’t stick to soil and is quite mobile. It is easily leached from the soil through rain.
ACKNOWLEDGEMENT
The University of Waikato Te Whare Wānanga o Waikato
Nitrogen fixation (NO2 → NH4+)
Nitrogen fixation is the conversion of nitrogen gas from a mostly unusable form to a form that can be used by plants. The conversion of dinitrogen gas through to ammonium is carried out in the nodules of clover plants by bacteria called rhizobia. There are other forms of nitrogen fixers, such as kōwhai trees – they also have bacteria in root nodules that can convert dinitrogen into nitrogen that can be used by plants.
Inage shows rhizobia nodules attached to roots of Vigna unguiculata.
ACKNOWLEDGEMENT
Dave Whitinger, CC BY-SA 3.0
Leaching to groundwater
Water can leave the land through a process called leaching, and this is where the water moves directly down through the soil profile and into groundwater.
And that’s probably the largest way that nitrogen will leave a farm is when you have got water in excess of what is a called the field capacity of the soil at that site, which is the amount of water that a soil can normally hold without leaching – basically, water starting to move down through it.
As that water moves, it can carry different chemicals with it as well and in particular carries negatively charged chemicals with it. Positively charged chemicals are held by the soil because soil is slightly negatively charged. One of the important forms of nitrogen is called nitrate, and that is negatively charged and so is repelled by soil, and you get that moving down with the water to groundwater and then onto surface waters like streams.
ACKNOWLEDGEMENTS
Professor Louis Schipper, The University of Waikato Te Whare Wānanga o Waikato
Denitrification (NO3- → N2O, N2)
Denitrification is a microbial process where microorganisms use nitrate, which is a form of nitrogen that is biologically available, and they convert it through back to nitrogen gas. So it is essentially the completion of the nitrogen cycle. In some ways, it’s the opposite to nitrogen fixation. So nitrogen fixation takes nitrogen gas out of the atmosphere and makes it biologically available, whereas denitrification converts that nitrogen back out of the soil back to nitrogen gas, completing the nitrogen cycle.
ACKNOWLEDGEMENTS
Professor Louis Schipper, The University of Waikato Te Whare Wānanga o Waikato
Soil nitrogen (N2) gas
Nitrogen gas makes up about 80% of the gases in the atmosphere and in the soil and can be fixed by specific microbes in conjunction with plants such as clover. Those plants can be eaten by cows or other animals and then excreted, which allows the fixed nitrogen to enter the rest of the ecosystem and start a cycle through other plants and animals.
ACKNOWLEDGEMENT
The University of Waikato Te Whare Wānanga o Waikato